The current position: Home > LATEST NEWS > Can starch and cellulose be used as flame retardants? Full interpretation of bio-based flame retardants >

Flame retardant, also known as flame retardant, fire retardant or fire retardant, is one of the important additives for synthetic polymer materials. Its function is to make synthetic materials have flame retardant, self-extinguishing and smoke suppression properties. In recent years, with the enhancement of people's awareness of ecological protection and environmental protection, green flame retardants have received more and more attention. Green flame retardant refers to green products with environmental protection in raw materials, production process, use and recycling. With the development of bio-based materials science, more and more scholars choose the raw materials of flame retardants in the field of bio-based materials. The reason why bio-based materials can be used as raw materials for flame retardants is that many bio-based materials have high carbon content and multi-hydroxy structure, which have excellent charring properties, such as lignin (Li), starch (ST), cellulose, chitosan (CS), cyclodextrin, etc.
一 Flame retardant principle of bio-based flame retardant The combustion reaction generally includes three elements: combustible, oxygen and a certain temperature, and none of them is indispensable. The action mechanism of flame retardants should be to inhibit the production of one or more elements during the combustion of materials, so as to prevent or slow down the combustion. The specific flame retardant mechanism of each flame retardant is different, but the basic principle of flame retardant is basically the same. Reducing the formation of combustible gas in the process of thermal decomposition and blocking the basic reaction in the process of gas combustion, absorbing the heat in the combustion zone, diluting and isolating the air also have a certain role in preventing combustion. Flame retardants can be divided into additive flame retardants and reactive flame retardants according to the application mode. Additive flame retardants are the main body of flame retardants, which are directly mixed with resin or rubber, convenient for processing and widely applicable; Reactive flame retardants are often bonded into the polymer chain as monomers, which have little impact on the properties of products and have lasting flame retardancy. Biobased flame retardants are mainly intumescent flame retardants. The flame retardancy principle of intumescent flame retardants depends on the formation of porous foam coke layer on the surface of materials. It is a multiphase system containing solid, liquid and gaseous products. At a lower temperature, non-bicyclic phosphorus in the compound produces phosphoric acid which can be used as a dehydrating agent; The water vapor generated by the reaction and the incombustible gas generated by the gas source make the molten system expand and foam. At the same time, polyols are dehydrated and carbonized to form inorganic substances and carbon residues, and the system is further expanded and foamed; When the reaction is nearly completed, the system gelatinizes and solidifies, and finally forms a porous foam carbon layer, which plays the role of preventing heat transfer, reducing the release of combustible gases and isolating oxygen, so as to achieve the purpose of flame retardancy. The flame retardancy of the carbon layer is mainly reflected in: making it difficult for heat to penetrate the condensed phase, preventing oxygen from entering the combustion area, and preventing the gaseous or liquid products generated by degradation from overflowing the material surface. 二 Common biological flame retardants 1.Lignin
Lignin is an amorphous aromatic polymer widely existing in plants, and its aromatic structure has a high carbon residue rate after decomposition. It exists in the cell wall of plants and forms the basic skeleton of plants together with cellulose and hemicellulose. It is a polyhydroxy aromatic compound that meets the requirements of carbon source as an intumescent flame retardant.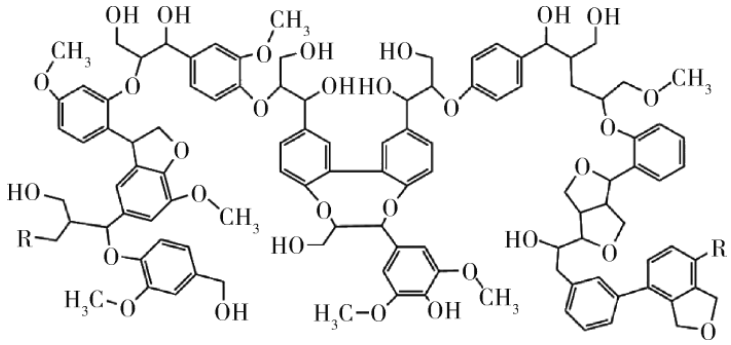
Lignin will break old bonds and form new bonds during combustion, and its pyrolysis process can be roughly divided into the following three stages: • The first stage is free water volatilization; • In the second stage, from about 120 ℃, the weak bond around the benzene ring breaks and the volatile components recombine; • In the third stage, when the temperature reaches 800 ℃, the benzene ring will be cracked, volatilized and polymerized into polynuclear aromatic compounds. With the further increase of temperature, the new aromatic compounds will undergo the polycondensation and carbonization process. It shows that lignin has high charring capacity at high temperature, and hardly chars during ordinary combustion. However, its structure contains a variety of functional groups, such as methoxy, alcohol-hydroxy, phenol-hydroxy, benzene, aldehyde, carbonyl, etc., which provides rich active sites for further chemical modification and is conducive to improving its flame retardancy. The combination of lignin with other flame retardants such as metal hydroxide and phosphorous compounds can further improve the flame retardancy. 2. Starch Starch is polymerized from glucose molecules and stored in cells in the form of starch granules. Starch is a polyhydroxy substance that can be cross-linked into carbon during combustion. It has the advantages of biodegradability, renewable and low cost, and is considered as a promising sustainable material.
Its thermal degradation can be roughly divided into the following three stages: • Physical dehydration mainly occurs. When the temperature reaches about 150 ℃, the crystalline water in the starch completely disappears; • Thermal decomposition and chemical dehydration of starch occur at about 300 ℃. On the one hand, condensation reaction occurs between hydroxyl groups to form ether bonds and dehydration, on the other hand, adjacent hydroxyl groups in the glucose ring will also be chemically dehydrated to form carbon-carbon double bonds or ring breaks, and the continuous temperature rise, the molecular chain breaks, forming a variety of aromatic structures; • Carbonization occurs at 500 ℃ and forms large aromatic conjugated rings. In the flame-retardant PLA system, it can act as a carbon source, which will release carbon dioxide and carbon monoxide during combustion. When combined with the acid source, the acid source can promote the dehydration and carbonization of starch, and the formed carbon layer can inhibit the escape of combustible gas and the exchange of thermal oxygen. The effective bio-based flame retardant prepared by using renewable and cheap potato starch as a bio-based charring agent not only promotes the development of green environmental protection flame retardant, but also greatly reduces the cost of flame retardant, which has very valuable practical value. 3. Cellulose Cellulose is a cell wall component. In short, cellulose is composed of glucose molecules β- Straight chain macromolecules linked by 1,4-glycosidic bonds, with the molecular formula of (C6H10O5) n (where n is the degree of polymerization), are widely distributed in nature and mainly exist in the cell walls of higher plants, bacteria, algae and fungi.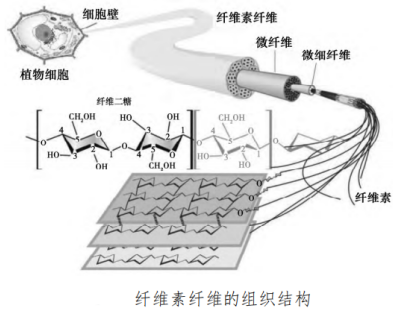
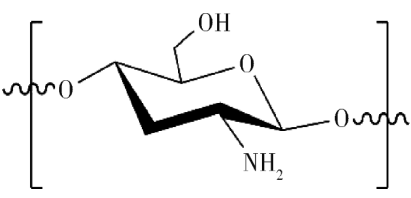
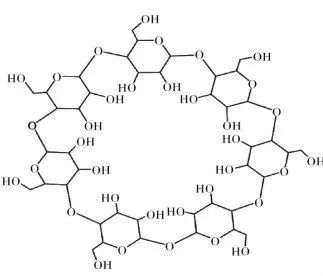
The thermal degradation process of cellulose can be roughly divided into the following four stages: • In the first stage, physical dehydration occurs at low temperature to remove crystalline water in cellulose; • In the second stage, chemical dehydration takes place at about 150 ℃ to generate water and dehydrated cellulose. The formation of water is conducive to accelerating the hydrolysis of glycosidic bonds and promoting the degradation of cellulose; With the increase of temperature; • In the third stage, thermal decomposition and carbonization reaction will take place from 240 ℃ to generate liquid product tar and carbon-containing intermediate products, while dehydrated cellulose will further react to generate carbon monoxide, carbon dioxide and water vapor; • In the fourth stage, aromatization and cross-linking of carbon occur above 400 ℃ to form coke slag. It is noteworthy that the reaction tends to generate tar and inhibit coke formation under high temperature conditions. However, rich modification technology is beneficial to improve the flame retardancy of cellulose at high temperature. The polyhydroxy structure of cellulose makes chemical modification an effective way to improve flame retardancy. Phosphorylation of cellulose is the most widely studied modification method at present, and esterification is the most common and simplest reaction. 4. Chitosan Chitosan (CS) is prepared by deacetylation of chitin, which has the advantages of renewable and good biocompatibility. CS is a positively charged natural aminopolysaccharide, which can be directly used as a char-forming agent for flame-retardant PLA composites. Under high temperature, ring opening reaction will occur, and the aromatic ring cross-linking structure will be formed by self-condensation in the matrix, that is, the carbon layer will be formed in the condensed phase, which is conducive to inhibiting the heat exchange in the matrix. At the same time, the amino group in CS is released into the gas phase in the form of NH3 during thermal decomposition, which can dilute the concentration of combustible gas on the one hand, and promote the formation of an expanded carbon layer, which has a better protective effect on the matrix than the ordinary carbon layer. The pyrolysis process of cyclodextrin can be divided into: • In the first stage, physical dehydration occurs at about 40 ℃ β- Crystalline water in CD; • In the second stage, thermal decomposition and carbonization reaction will occur at 260 ℃ to generate carbon dioxide gas and residual carbon; • In the third stage, when the temperature reaches 400 ℃, the residual carbon will undergo slow thermal degradation. β- In addition to the multi-hydroxyl structure that can be used for carbonization, CD also contains more active primary and secondary hydroxyl groups. It can be modified by esterification, crosslinking and chemical modification to improve its flame retardance. CD has an outer hydrophilic and inner hydrophobic cavity structure, which enables it to form inclusion complexes with a variety of molecules, providing more space for its modification. CD thermal degradation will form a large amount of carbon residue, which can be used as carbon source in intumescent flame retardant system or as embedding agent of phosphorus compound to improve its low binding effect. Bio-based flame retardant is a new variety. Many bio-based raw materials were originally used in medicine, food and other fields, and there are no special chemical grade products. Many bio-based materials or chemicals can be properly treated to have the characteristics of being used as flame retardants. Although the bio-based flame retardant is still in the research and development stage of the laboratory, with the research and development of bio-based raw materials in the field of additives, the bio-based flame retardant raw materials that can be used in industrial production will be developed in the future.